IV) Computer Assisted Drug Design
B) Investigation of Target Compounds in Connection with Drug Design
3. Investigation of Artemisinin – a Traditional Chinese anti-Malaria drug
Malaria is a disease that causes a lot of suffering, mainly in the third world. It causes the death of at least 1 million people each year. Malaria is a growing threat because of the emergence of multi-drug resistant strains of the parasite that causes the disease. There is a large need for novel approaches to the anti-malarial chemotherapy. Artemisinin (qinghaosu), is just such a new approach with a molecular mechanism of action separate from the established drugs. Artemisinin is a compound isolated from the plant Artemisia annua, which leaves have been used for 2000 years by Chinese herbalists. The last two decades artemisinin has received a lot of attention, and the use of it is spreading out of Asia. We studied the mechanism of artemisinin and derivatives using quantum chemical methods.
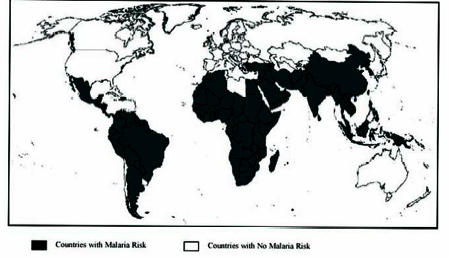
Figure 1. Malaria endemic countries year 2000 {Centers for Disease Control and Prevention}
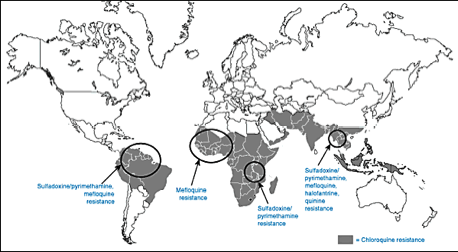
Figure 2. Distribution of resistance to available anti-malarial drugs year 2001 {Centers for Disease Control and Prevention}
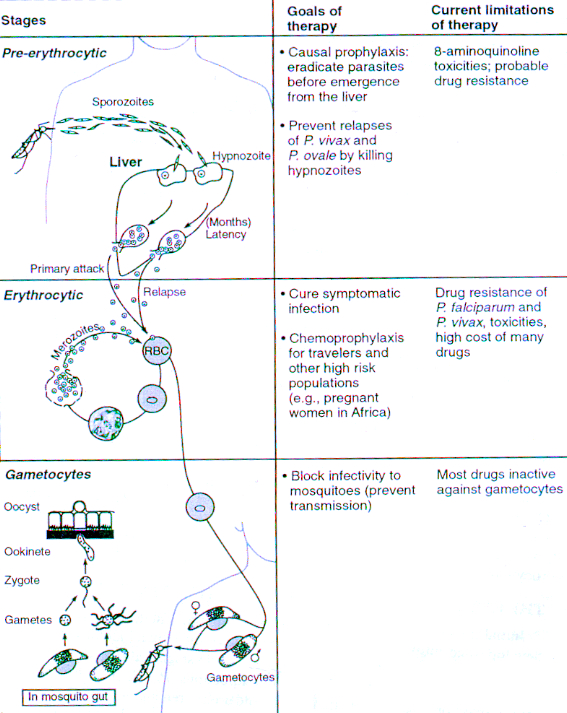
Figure 3. Life cycle of Plasmodium parasites; Antimalarial chemotherapy [2]
5. Generation and Toxicity of Acrylamid
From time to time, special chemical compounds are in the focus of public interest because they have found to be particularly useful as a new health conserving drug or particularly dangerous in view of their toxicity. Such a compound is acrylamide, the name of which is today even familiar to the general audience. Acrylamide entered the media when first reports emerged in newspapers worldwide in the April of 2002 that relatively high acrylamide concentrations had been found in food cooked at higher temperatures [1]. This included for example french fries, roasted potatoes, coffee, bread and chips. At this time it was already known that acrylamide can be considered to be carcinogenic [2] and therefore the news reports received a strong echo and many concerns from physicians, health organizations, and public health administration. For the next twelve months extensive reports on the potential danger of food containing acrylamide could be found in all media.
Government agencies in several European countries and the USA started programs to determine the concentration of acrylamide in all kinds of cooked, broiled or grilled food. One after the other report appeared, which seemed to enlarge the danger of acrylamide [3 - 5]. However there were also reports, which played down the danger of acrylamide. On the basis of a statistical survey of the eating habits and health conditions of about 1500 adults borne between 1918 and 1942, the claim was made in the beginning of 2003 that the toxicity of acrylamide was largely exaggerated and that this compound cannot lead to cancer [6]. Clearly, the time and methods used for this survey can be criticized and any conclusion with regard to the toxicity or non-toxicity of acrylamide seems to be too early. Therefore, any scientific approach to the acrylamide problem should start from the well-established facts:
- It is proven that acrylamide is formed at temperature of 180°C and higher in cereals, french fries, pancakes, biscuits and various other food. This fact implies that any cooking, broiling or grilling of food at lower temperatures would not lead to the acrylamide problem. However, this consideration leaves out the fact that large amounts of pre-prepared food reach the market and are consumed by the public. This does not only include food consumed in restaurants, but also commercially sold food in supermarkets.
- Prepared food such as cereals, crackers, biscuits, and toasts contains acrylamide in various concentrations. Industry either claims that the acrylamide concentrations measured are of no relevance for health conditions or that the acrylamide results from different sources. However production details are not disclosed and there is little tendency to change these details.
In this situation, there is the necessity of approaching the problem from a strictly scientific and preferentially chemical side to clarify basic facts. We make a contribution in this direction by pursuing the following goals and objectives:
- In this investigation the chemical, biochemical, and toxiological background of the acrylamide problem is summarized with the goal of presenting the facts and leaving out the speculations.
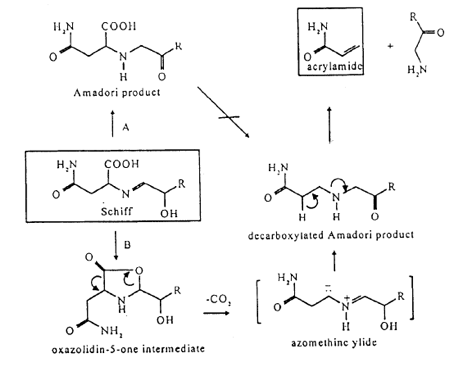
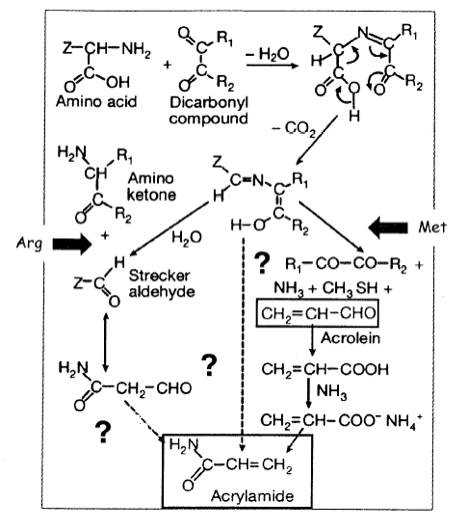
- Acrylamide is known to be formed in the Maillard reaction, which is a key reaction in food chemistry [7 - 10]. Although there are literally hundreds of investigations on the Maillard reaction, a detailed description of the mechanism of this reaction including possible intermediates and the energetics, has, to the best of our knowledge, never been given. Since by trapping experiments and kinetic measurements this information is difficult to obtain, we use quantum chemical methods to calculate rather than to measure energy data, which throw a light on the exact mechanism of the acrylamide generation.
- If acrylamide may be carcinogenic, its consumption in form of a product to the normal food must lead to the generation of radicals, which are known to be cancer-causing species. Acrylamide could lead to radicals in one of three different chemical ways. First it could form radicals by CH or NH bond cleavage. We will investigate this possibility by determining the bond strength of CH and NH bonds. Secondly, heavy atom bonds could be broken thus leading to radicals such as the vinyl radical, the NH2 radical or the C(=O)NH2 radical. We will discuss also this possibility. Finally, it could be that by the addition of molecular oxygen to the double bond or by H-abstraction peroxyl radicals are formed, which are known to be toxic.