IV) Computer Assisted Drug Design
A) Computer Assisted Drug Design
3. Quantum Chemical Investigation of the Interaction Mechanism between Naturally Occurring Enediynes and DNA
Certain microorganisms are found to produce the natural enediynes dynemicin, calicheamicin, esperamicin, etc. [1-4] These enediynes have the ability to destroy the DNA of toxic bacteria and viruses in a three-step process. First, a delivery system helps the enediyne to dock into the minor groove of the DNA helix. Then, a molecular trigger device causes the actual enediyne unit of the natural compound to undergo a Bergman cyclization [5] and to form a highly reactive p-didehydrobenzene biradical. This biradical represents a biological warhead that attacks and destroys DNA thus causing bacteria cell death. [6]
Literally hundreds of research projects have been devoted to understanding the biological activity of the naturally occurring enediynes, among them many quantum chemical investigations. [7] Common to most of these investigations is the fact that only the warhead (without triggering system and docking tail) of the natural enediynes is investigated and the strong toxicity of the enediynes is completely ignored. Little or no work has been done to investigate the interaction of naturally occurring enediynes (dynemicin A, calicheamicin, ) with DNA. The work published, although important, is based on MM (molecular mechanics) calculations [8,9] and therefore cannot describe the actual interactions of the endiynes with DNA which involves, besides nonbonded interactions, a series of bond forming and bond breaking steps.
We have recently investigated the reaction of dynemicin A with DNA in aqueous solution using quantum chemical in combination with MM methods. [10] Triggering, biradical forming, and H-abstraction reactions were carried out for dynemicin docked into DNA thus leading for the first time to a useful description of the energetics and the stereochemical implications of the DNA-dynemicin interactions. [10] Some evidence was gathered that there is an equilibrium between intercalation and edge-on insertion of dynemicin into the minor groove and that the actual biological activity of dynemicin is developed in the insertion rather than the intercalation mode, which could have far-reaching consequences for the design of a suitable non-toxic antitumor drug based on naturally occurring dynemicin. These investigations were however incomplete and thus still questionable due to some serious shortcomings imposed on our model because of insufficient computational resources. In particular: a) Neutral dynemicin rather than its anion as it exists in aqueous solution must be considered; b) DNA was modeled by a decamer segment possessing a total of 22 negative charges; c) The DNA fragment was geometry optimized only in the gas phase where MM methods could be used--these were originally set up for proteins; d) The optimization of neutral dynemicin A was limited to the gas phase using DFT and a relatively small basis set; e) Dynemicin A was docked into DNA using a rigid docking protocol; [11] f) Water as a solvent was not explicitly included; g) Counterions were not considered; h) The resulting complex was not optimized.
We will improve this model substantially considering the following: 1) Anionic dynemicin A will be optimized in the gas phase utilizing a VDZ+P basis set to obtain more reliable energetics for the biradical formation. 2) The DNA fragment will be optimized using a CHARMM[12] force field for nucleic acid modeling. 3) The DNA-enediyne complex will be solvated with TIP3P (transferable intermolecular potential with three parameters) water.[13] 4) The system charge will be neutralized through the inclusion of a suitable number of counterions. 5) DNA, solvent and counterions, with dynemicin embedded into the DNA segment will be minimized. 6) An initial MD simulation [14] will be carried out to equilibrate the environment, then 7) a hybrid QM/MM optimization of the equilibrated structure, and finally 8) a hybrid QM/MM optimization of the full reaction profile of the biradical formation of dynemicin A in the minor groove of DNA. The most expensive part of our investigation is the QM calculation of energy and energy gradient, whereas the MM part and ChemShell [15] calculations are essentially negligible.
In a further step we will focus on the chromoproteins, an enediyne class, for which the nonpetidic enediyne cromophore is complexed by a protein that acts as a carrier and stabilizer for the chromophore. Our studies will include the necarzinostatin, kedarcifin, C1027, and maduropeptin chromophore where we will first consider just the chromophore and later use the more realistic model of the chromophore sitting in the cleft of the corresponding apoprotein, which will increase significantly the computational load. - In each case, we have to consider six different steps of enediyne-DNA interactions: a) Recognition step; b) Docking of the enediyne to DNA; c) Triggering of the endiyne; d) Bergman reaction in the docked/undocked situation; e) H-abstraction from DNA by the biradical formed in the Bergman reaction; f) Reactions leading to DNA (single or double-) strand fission. Apart from this we will investigate in the additional step of how an enediyne such as calicheamicin can damage the DNA repair enzyme poly(ADP-ribose)polymerase thus hindering repair of DNA. It is known that cell apoptosis is caused by enediynes via a two-pronged attack against both the cell nucleus and the repair proteins.
The overall-goal of our research is to describe the recognition step, to determine the preferred docking site, to clarify whether there is a competition between intercalation or insertion mode for the smaller enediynes (calichemicin and esparamicin will always prefer the insertion mode because of their carbohydrate tails), to elucidate the stereochemistry of the docking process (there are 19 stereogenic centers in calicheamicin g1I, two of which are placed in the headgroup), to determine energetics and stereochemistry of the triggering step and the biradical formation, and to clarify preferences for H-abstraction. Especially, we will consider questions such as: Can the triggering of an enediyne be made dependent on the cell type, normal or cancer? What reaction sequence is started by H abstraction so that DNA fission occurs?
References
- [1]Enediyne Antibiotics as Antitumor Agents; Borders D.B.; Doyle T.W., Eds.; Marcel Dekker: New York, 1995.
- [2]Thorson, J.S; Sievers, E.L.; Ahlert, J.; Shepard, E.; Whitwam. R.E.; Onwueme, K.C.; Ruppen, M., Understanding and exploiting nature's chemical arsenal: The past, present and future of calicheamicin research Curr. Pharm. Design, 2000, 6, 1841-1879.
- [3]Shen, B.; Liu, W.; Nonaka, K., Enediyne Natural Products: Biosynthesis and Prospect towards Engineering Novel Antitumor Agents. Current Med. Chem. 2003, 10, 2317-2325.
- [4]Jones, G.B.; Fouad, F.S., Designed Enediyne Antitumor Agents, Current Pharm. Design, 2002, 8, 2415-2440.
- [5]Bergman, R.G., Reactive 1,4-dehydroaromatics, Acc. Chem. Res., 1973, 6, 25-31.
- [6]Marquardt, R.; Balster, A.; Sander, W.; Kraka, E.; Cremer, D.; Radziszewski, J.G., p-Benzyne, Angew. Chem.,Int. Ed. 1998, 37, 955-958.
- [7]See e.g., Klein, M.; Walenzyk, T.; Konig, B. Electronic effects on the Bergman cyclisation of enediynes. A review. Collection of Czechoslovak Chem. Comm. , 2004, 69, 945-965, and references cited therein.
- [8]Langley, D.R.; Doyle, T.W.; Beveridge, D.L. The Dynemicin Dna Intercalation Complex - A Model Based On Dna Affinity Cleavage And Molecular-Dynamics Simulation, J. Am. Chem. Soc., 1991, 113, 4395-4403.
- [9]Cardozo, M.G.; Hopfinger, A., The Dynemicin Dna Intercalation Complex - A Model Based On Dna Affinity Cleavage And Molecular-Dynamics SimulationJ. Biopolym., 1993, 33, 377-388.
- [10]Tuttle, T.; Kraka, E.; Cremer, D., Docking, Triggering, and Biological Activity of Dynemicin A in DNA: A computational Study. J. Am. Chem. Soc. 2005, 127, 9469-9484.
- [11]Autodock: Morris, G.M.; Goodsel, D.S:; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J., Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function J.Comp. Chem. 1998 19, 1639-1662.
- [12]Brooks, B. R.; Bruccoleri, R. E.; Olafson, B. D.; States, D. J.; Swaminathan, S.; Karplus, M., Charmm - A Program For Macromolecular Energy, Minimization, And Dynamics Calculations. J. Comput. Chem. 1983, 4, (2), 187-217.
- [13]TIP3P: Jorgensen, W.L.; Chandrasekhar, J.; Madura,J.D.; Impey, R.W.; Klein, M.L., Comparison of simple potential functions for simulating liquid water, J. Chem. Phys., 1983, 79, 926-935.
- [14]The MD simulations will be performed with CHARMM (Ref. 12), and DL_POLY (Ref.18).
- [15]Sherwood, P., et al., QUASI: A general purpose implementation of the QM/MM approach and its application to problems in catalysis. Theochem-J. Mol. Struct. 2003, 632, 1-28.
- [16]COLOGNE2005, Kraka, E.; Gräfenstein, J.; Filatov, M.; He, Y.; Gauss, J.; Wu, A.; Polo, V.; Olsson, L.; Konkoli, Z.; He. Z.; Cremer D., University of the Pacific, Stockton, 2005.
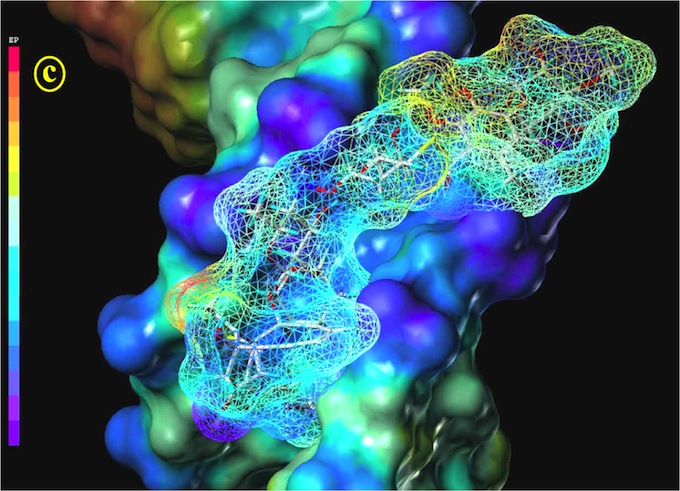
Figure 3: Calicheamicin in its gas-phase B3LYP/3-21G geometry docking into the 9mer-B-DNA d(CACTCCTGG-d(CCAGGAGTG). The DNA is represented by a solid Connolly surface, the alicheamicin molecule, by a stick model and a mesh Connolly surface. The hydrogen-bonding property (red: H donors, blue: H acceptors) is mapped on both Connolly surfaces.
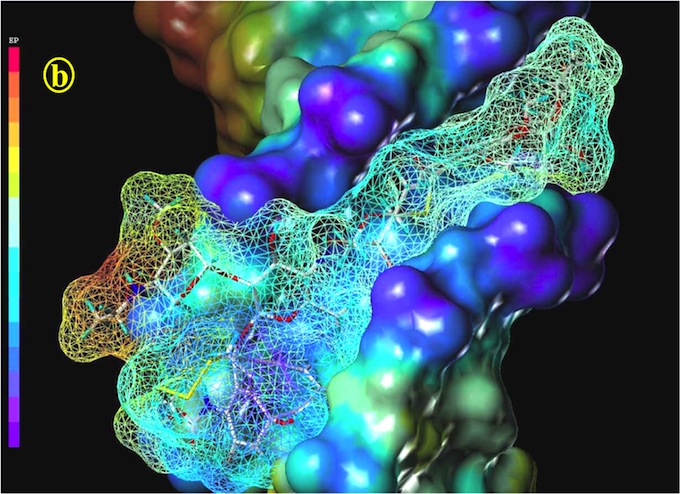
Figure 4: Calicheamicin in a refined geometry, taking solvent effects into account, docking into the 9mer-B-DNA d(CACTCCTGG-d(CCAGGAGTG). Representation as in Fig. 3
4. Interactions between DNA and Rhenium complexes – Development of a Rhenium-based Antitumor Drug
(Project leader: Dr. D. Cremer)
One of the most effective antitumor drugs is cis-platin (cis-diamminedichloroplatinum(II)). However, chemotherapy based on this compound is accompanied with considerable side effects (kidney damage, nerve damage, hair loss, etc.). Therefore, one is searching for alternatives to cis-platin. Promising are dirhenium compounds, which have been found to be also cytotoxic to tumor cells. For the purpose of finding the most suitable dirhenium compounds, one has to understand their interactions with DNA via base-binding. Relativistic quantum chemical calculations are used to accurately describe the many possible binding modes between dirhenium compounds and adenine and guanine as the most important DNA bases. The results are used to guide synthesis and testing of the dirhenium compounds.
5. Determination of NMR chemical shifts and NMR spin-spin coupling constants of Calicheamicin
With the design of new non-toxic enediyne-based antitumor antibiotics there is the need to think of the experimental realization of these drugs. Whereas on the synthetic side there is already a solution as for the synthesis of these compounds, there is increased need to identify structure and conformational specifics of enediynes (including the new drugs) by NMR. For calicheamicin itself NMR chemical shifts and NMR spin-spin coupling constants have been published and partially assigned. Also NMR has been used with some success to follow the reactive behavior of the enediynes in the cell. These experimental efforts can be supported and improved by quantum chemical calculations.
Using the software developed by members of PCTC, we will carry out a detailed description of calicheamicin in terms of NMR chemical shifts and NMR spin-spin coupling constants using a strategy taken from the experimental investigation of the compounds. Due to the extended structure of the molecule, it is sufficient to partition the molecule in segments and to investigate the segments separately. For example, a natural segmentation of calicheamicin used by experimentalists leads to the headgroup and rings A, B, C, D, and E. In this way, it is possible to use larger basis sets for the calculation of the NMR parameters. Especially, a reliable calculation of the Fermi contact (FC) part of the spin-spin coupling constants requires basis sets decontracted and augmented in the core region to accurately describe the spin density at the core.
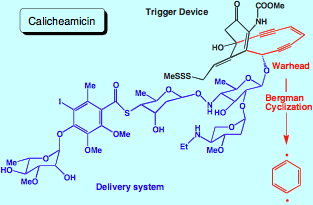
Figure 1. Delivery system, triggering device, and warhead of the naturally occurring enediyne calicheamicin.
